.png)
Off-the-shelf systems force your process to fit the equipment. We take the opposite approach. Our engineer-to-order bioprocessing systems are designed around your workflow, your scale, and your goals, from benchtop TFF to full-scale continuous manufacturing. Powered by next-generation precision pumps, valves, and flow control, every system is software agnostic and built to integrate seamlessly into your facility.
.png)
Every biopharma process has its own pressure curves, flow dynamics, and critical quality attributes. Off-the-shelf bioprocessing skids are engineered to satisfy the broadest market — not your specific application. The gap between "close enough" and "right for you" is where failures happen.
Skids designed for generic workflows force your team to re-engineer process parameters every time you scale. What works at 50L fails at 500L, not because of your science, but because the equipment was never designed with your process in mind.
Mixing components from multiple suppliers creates extractables and leachables complexity that industry bodies describe as making direct vendor comparison "almost impossible." Five suppliers means five validation packages and five fingers pointing elsewhere when something fails.
Process intensification, continuous manufacturing, and N-1 perfusion strategies demand flexibility that fixed-configuration skids simply do not provide. When your process evolves, and it will, standard equipment forces costly redesigns, revalidation, and often complete replacement.
Industry-wide, batch failure costs run to tens of millions annually per organisation. A single failed clinical-scale run is a major programme setback beyond just the lost materials.
We do not sell you a system and ask you to adapt. We engineer around your process from the first P&ID to final commissioning, so your equipment fits your science, not the other way around.
Discuss Your Process →Every bioprocessing system starts with your process goals. We work with you through every milestone, from initial concept through full commissioning and ongoing support, so your system performs exactly as intended from day one.
We define your process requirements, flow rates, pressures, and integration needs together.
Full P&IDs, component selection, control architecture, and design qualification (DQ).
System fabrication using precision single-use components, custom fluid paths, and overmolded assemblies.
Factory acceptance testing (FAT), installation qualification (IQ), and operational qualification (OQ).
Ongoing maintenance, consumable supply, system upgrades, and dedicated engineering support.
Every system we build is designed to integrate with your existing control infrastructure. Whether you run DeltaV, Rockwell, Siemens, Wonderware, or any other SCADA/PLC platform, our automation-ready architecture slots in seamlessly. No proprietary lock-in, no costly middleware. Your system, your software, your choice.
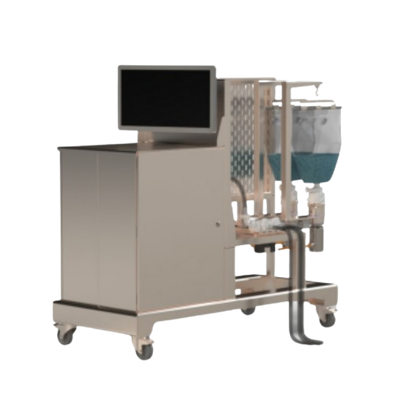
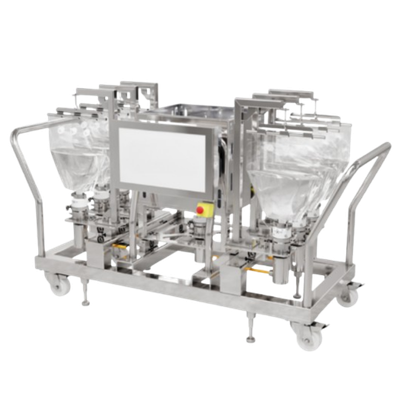
These are examples of the bespoke bioprocessing systems we engineer for pharma and biotech manufacturers worldwide. Every system is designed around your unique process requirements. These represent starting points, not fixed configurations.

A modular, multi-unit bioprocess system designed for Pharma 4.0 manufacturing environments. Featuring a reconfigurable process wall interface, this system unifies diverse bioprocessing operations for both batch and continuous workflows. It handles everything from large-scale monoclonal antibody purification to personalised cell therapy manufacturing, scaling from laboratory volumes to thousands of litres.
.png)
Engineered to work in conjunction with continuous processing residence time units, this viral inactivation system feeds the surge vessel, mixes to ensure product homogeneity, and pushes product into the residence time unit. Custom mixing options (top, bottom, or ratio) adapt to your specific process needs, with pulsation damping and precise feed control safeguarding sensitive biologics throughout the inactivation step.

A multipurpose buffer dilution system capable of performing simple dilution-based buffer preparation in batch or continuous mode, or acting as a buffer blending system combining four or more stock solutions into complex compound buffers to power entire downstream processes. Precision inline dilution eliminates the need for external surge vessels, reducing cleanroom footprint and operating costs significantly.
These are examples, not the limit of what we build. Whether it's chromatography, depth filtration, formulation, fill-finish, or something entirely unique, our engineering team designs custom bioprocessing systems for any downstream or upstream application.
Discuss Your Custom System →Every custom bioprocessing system is powered by next-generation precision fluid control components. Purpose-engineered building blocks designed to work as an integrated system, giving you unmatched control over flow, pressure, and process integrity.
.png)
A single-use positive displacement diaphragm pump delivering low-shear, high-precision fluid handling. Flow rates from 1 mL/min to 200+ L/min with pressures up to 6 bar — ideal for cell therapy, mRNA, and sensitive biologics.
View PIXER Pump →.png)
A compact single-use radial diaphragm valve delivering precise flow control with a smaller, cleaner footprint. Also available as Vannefold™ manifold configurations for multi-port applications.
View Single-Use Valves →.png)
A single-use pinch valve offering sterile, dependable flow control without added complexity. Available in manual, pneumatic, and motorised eMaxion™ configurations for full automation integration.
View Pinch Valves →.png)
A single-use check valve ensuring reliable one-way flow to protect products, equipment, and process integrity. Spring-free design with clean straight-through flow path and gamma-stable construction.
View Check Valve →.png)
A single-use 2.5D design bio container combining 3D volume capacity with 2D fluid management. Engineered for optimal mixing, drainability, and integration into custom bioprocessing systems.
View Single-Use Bags →.png)
Engineered to your exact process requirements, our single-use fluid path assemblies integrate tubing, connectors, and LSR overmolded fittings into a validated, leak-free system — ready to drop into any upstream or downstream process.
View Flow Paths →You're not just buying a bioprocessing system. You're gaining a precision-engineered, process-driven partner who works with you from first concept to ongoing production support.
Every system begins with your process goals: your flow rates, pressures, volumes, and workflow. We engineer around your requirements, not around a fixed product catalogue. The result is a system that fits your process exactly, with no compromises.
Pumps, valves, fluid paths, bio containers, and overmolded assemblies. We supply the components and build the system. One partner, unified control, and simplified validation and commissioning.
Our systems are powered by the latest generation of single-use fluid control technology, including ultra-low shear diaphragm pumps, precision pinch valves, and radial diaphragm valves built for consistent, repeatable bioprocess performance.
We combine modular architecture with process-specific customisation. Reconfigurable process walls, scalable volumes, and interchangeable modules give you the flexibility to adapt as your manufacturing evolves, without replacing the entire system.
Every system is built to evolve with your operation, from process intensification to Pharma 4.0 integration. Automation-ready design means your system can integrate with your existing SCADA, PLC, or DCS platform and be upgraded as your needs grow.
From concept design through commissioning and beyond, we support every stage of your project. Ongoing consumable supply, system upgrades, and direct access to our engineering team ensures your system performs long after handover.
Frequently Asked Questions
A custom bioprocessing system, also called a bespoke bioprocess skid or engineer-to-order (ETO) system, is a purpose-built process platform designed around your specific manufacturing requirements. Unlike off-the-shelf equipment, custom single-use bioprocessing systems are fully engineered to match your exact flow rates, pressures, hold volumes, process steps, and facility constraints. We design and build bespoke single-use systems for applications including tangential flow filtration (TFF/UF/DF), viral inactivation, inline buffer dilution, chromatography, depth filtration, and formulation for biopharmaceuticals including monoclonal antibodies, cell and gene therapies, mRNA, vaccines, and ADCs.
We design and build a comprehensive range of single-use bioprocess systems including benchtop and pilot-scale TFF skids, modular multi-unit downstream processing systems, viral inactivation feed and hold systems, inline buffer dilution and blending skids, fill-finish preparation systems, and custom chromatography automation systems. Every system is built around next-generation precision fluid control components including PIXER® single-use diaphragm pumps, VannX™ diaphragm valves, ARTēVA® pinch valves, and SoloCheck™ check valves. If your application is not listed, contact our engineering team.
Yes. Every system we build is fully automation-agnostic and integrates with your existing control infrastructure. Compatible with all major SCADA, PLC, and DCS platforms including DeltaV, Rockwell Automation (Allen-Bradley), Siemens S7/TIA Portal, AVEVA (Wonderware), and Inductive Automation Ignition. There is no proprietary software lock-in. Systems are delivered automation-ready with standard I/O mapping. If you have an existing site standard, we design to it.
Our engineer-to-order (ETO) process follows five milestones: (1) Concept Design - defining process requirements and system architecture with your team; (2) Detailed Design - full engineering package including P&IDs, 3D layouts, and Design Qualification (DQ) documentation; (3) System Build - in-house manufacturing and assembly; (4) FAT, Installation and Commissioning - including IQ and OQ protocols; (5) Ongoing Support - consumable supply, spare parts, and field service. Every phase is GMP-documented. Contact us to begin.
Lead times vary with system complexity and scope. Standard benchtop and pilot-scale systems typically deliver within 12 to 20 weeks from design sign-off. More complex modular or multi-unit production systems may require 20 to 36 weeks. Because core fluid control components are manufactured in-house, there is significantly more control over supply chain and delivery timelines than suppliers reliant on third-party procurement. Contact our team early in your planning to align lead times with your programme schedule.
Yes. Our systems support both traditional batch processing and continuous bioprocessing and process intensification (PI) strategies. Modular multi-unit systems feature reconfigurable process wall interfaces adaptable for perfusion, semi-continuous, or fully continuous downstream operations. As the industry moves toward continuous manufacturing to improve yield, reduce hold volumes, and accelerate cycle times, our systems are built to evolve with your manufacturing strategy.
We provide full qualification support across the system lifecycle, aligned with FDA 21 CFR Part 211, EU GMP Annex 15, and ICH Q7/Q10 requirements. This includes Design Qualification (DQ), Factory Acceptance Testing (FAT), Installation Qualification (IQ), and Operational Qualification (OQ). All systems are delivered with a complete GMP documentation package including material traceability, calibration certificates, and P&ID as-built drawings, ready to support your validation master plan (VMP) and regulatory submissions.
Standard equipment forces your process to adapt to fixed configurations. Our engineer-to-order approach does the opposite: every system is designed around your process goals, flow requirements, and facility footprint. Systems are powered by proprietary precision fluid control components including our PIXER® single-use diaphragm pumps, which use Polymorphic Pump Technology to deliver ultra-low shear, pulsation-free fluid transfer critical for preserving shear-sensitive biologics including viral vectors, mRNA lipid nanoparticles, and protein-based therapeutics. Combined with custom single-use assemblies and LSR overmolded connections, you get a fully integrated, single-source system.
Yes. As both system integrator and component manufacturer, we supply all single-use consumables used in your system including PIXER® pump heads, VannX™ valve diaphragms, single-use biocontainers, and custom fluid path assemblies. This single-source model simplifies procurement, guarantees compatibility, eliminates multi-vendor qualification overhead, and removes the risk of substitute components entering your validated process. We also offer consignment stock and framework supply agreements for high-volume operations.
Our systems cover a broad range of downstream processing and formulation applications: tangential flow filtration (TFF/UF/DF), viral inactivation and viral clearance, inline buffer dilution and buffer preparation, automated chromatography, depth filtration, drug substance formulation, and fill-finish preparation. Optimised for monoclonal antibodies (mAbs), bispecific antibodies, antibody-drug conjugates (ADCs), cell and gene therapy products (lentiviral vectors, AAV, CAR-T), mRNA and lipid nanoparticle (LNP) formulations, recombinant proteins, vaccines, and biosimilars.
Absolutely. Our modular architecture is purpose-designed for seamless scale-up from benchtop and pilot scale through to full GMP clinical and commercial manufacturing. The same PIXER® diaphragm pump components used at development scale are available at production scale, meaning your process parameters including flow rates, shear profiles, and hold times transfer directly without re-characterisation. This materially reduces tech transfer risk, accelerates clinical timelines, and maintains product consistency across scales. Our benchtop TFF system supports working volumes from 30 mL with modular upgrade paths enabling scale-up without redesigning your process.
Whether you need a complete bespoke bioprocessing system, a single-use process skid for a specific unit operation, or engineering consultation on your system architecture — our team is ready to help.